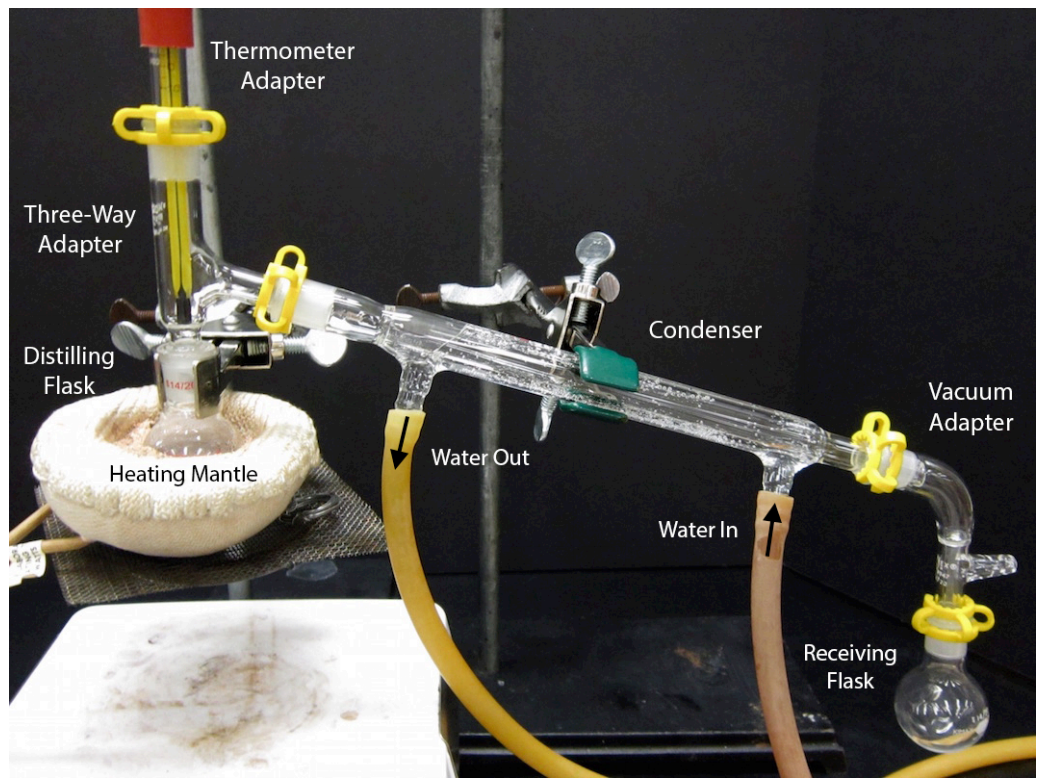
What Is The Role Of Safety Tube In Vacuum Distillation . A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This separation technique is based on variations in the. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. Why is a vacuum distillation unit needed? Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. What is the difference between atmospheric and vacuum distillation? What is vacuum distillation method? A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill the compound (or. The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within.
from chem.libretexts.org
This separation technique is based on variations in the. Why is a vacuum distillation unit needed? A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. What is the difference between atmospheric and vacuum distillation? What is vacuum distillation method? Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill the compound (or.
5.2C StepbyStep Procedures Chemistry LibreTexts
What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. Why is a vacuum distillation unit needed? What is the difference between atmospheric and vacuum distillation? What is vacuum distillation method? A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill the compound (or. This separation technique is based on variations in the. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation.
From tnlab.com
ShortPath Distillation System 2000ml 2L Essential Oil Distillation What Is The Role Of Safety Tube In Vacuum Distillation What is the difference between atmospheric and vacuum distillation? This separation technique is based on variations in the. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. What is vacuum. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. What is the difference between atmospheric and vacuum distillation? The aim of present. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.coursehero.com
[Solved] Organic Chemistry Laboratory Activity Data/Procedure for What Is The Role Of Safety Tube In Vacuum Distillation Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.youtube.com
ESSENTIAL OIL DISTILLATION YouTube What Is The Role Of Safety Tube In Vacuum Distillation Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. What is vacuum distillation method? What is the difference between atmospheric and vacuum distillation? Inspect every piece of glassware to be. What Is The Role Of Safety Tube In Vacuum Distillation.
From micoope.com.gt
Distillation Apparatus Diagram With Full Process And Lab, 44 OFF What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. A vacuum distillation is used when the boiling point of the compound (or. What Is The Role Of Safety Tube In Vacuum Distillation.
From slideplayer.com
Safety Slide Vacuum Reduced pressure used in HiVac drying, vacuum What Is The Role Of Safety Tube In Vacuum Distillation Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. What is the difference between atmospheric and vacuum distillation? Why is a vacuum distillation unit needed? A vacuum distillation is. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.youtube.com
Distillation The Operation of a Water Still YouTube What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. What is vacuum distillation method? Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Inspect every piece of glassware to be. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.scientificglassservices.co.uk
Selecting Laboratory Glassware for Distillation Scientific Glass Services What Is The Role Of Safety Tube In Vacuum Distillation A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. What is the difference between atmospheric and vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. This separation technique is based on variations in the. A vacuum distillation is used when the boiling point. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.electronicpro.co.za
1000mL 24/29 Glass Vacuum Distillation Extraction Distilling Apparatus What Is The Role Of Safety Tube In Vacuum Distillation Why is a vacuum distillation unit needed? Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. What is vacuum distillation method? A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.labsupply2u.com
Water Distillation Unit Malaysia Lab Supplies What Is The Role Of Safety Tube In Vacuum Distillation A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill the compound (or. Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. What is vacuum distillation method? Vacuum distillation is a process of purifying substances. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.youtube.com
Animated Water Distillation process diagram in PowerPoint / Chemistry What Is The Role Of Safety Tube In Vacuum Distillation The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. What is the difference between atmospheric and vacuum distillation? A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. What is vacuum distillation method? Why is a vacuum distillation unit needed? A vacuum distillation. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.numerade.com
Consider the Piping and Instrumentation Diagram (P ID) for Benzene What Is The Role Of Safety Tube In Vacuum Distillation What is vacuum distillation method? The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.diytrade.com
10L Vacuum Rotary Evaporator with SUS304 Water/Oil bath/Vacuum What Is The Role Of Safety Tube In Vacuum Distillation Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. The aim of present. What Is The Role Of Safety Tube In Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry What Is The Role Of Safety Tube In Vacuum Distillation A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Inspect every piece of glassware to be used with the vacuum distillation, checking for stars, cracks, or other. Why is a vacuum distillation unit needed? A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog What Is The Role Of Safety Tube In Vacuum Distillation Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in. What Is The Role Of Safety Tube In Vacuum Distillation.
From www.simplepharmanotes.com
Steam Distillation. What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The aim of present study is to investigate possible accidental risks occurring in vacuum distillation column of oil refining within. Inspect every piece of glassware to be used with the vacuum distillation, checking for. What Is The Role Of Safety Tube In Vacuum Distillation.
From solutionpharmacy.in
Steam Distillation How it Works? What Is The Role Of Safety Tube In Vacuum Distillation What is vacuum distillation method? Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. Why is a vacuum distillation unit needed? A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill. What Is The Role Of Safety Tube In Vacuum Distillation.
From sigmaglassindia.com
Glass Reactor, Pilot Plant Reactor , Jacketed Reactor, Kilo Lab What Is The Role Of Safety Tube In Vacuum Distillation This separation technique is based on variations in the. Vacuum distillation is a process of purifying substances that are difficult to distill at normal pressures, or to save time or energy. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t >150 o c) in order to distill the compound (or.. What Is The Role Of Safety Tube In Vacuum Distillation.